With the publication of FSSC 22000 Version 5, and the revision of ISO 22000: 2018, FSSC 22000 has produced a new FSSC 22000 Version 5 FSMA PCHF addendum. The role of the addendum is to help companies understand how they can integrate the requirements of the US FDA FSMA regulations into their FSSC 22000 Food Safety Management system, and use the FSSC 22000 risk-based audit as a means of sharing information relating to the control of hazards up and down the supply chain, enhancing transparency and contributing to compliance. It is anticipated that the revised FSSC 22000 FSMA Addendum will help companies all over the world be aware of, and implement, the appropriate expectations of the US FDA FSMA regulations.

The Addendum is accompanied by a revised gap analysis of the FSSC 22000 V5 requirements (Category C – Manufacture of Human Food) against the requirements of the FDA Preventive Controls for Human Food rule. This shows how well the two sets of requirements align at the same time as providing an explanation of the differences in the terminology used.
Jacqueline Southee, FSSC North America Representative will provide more background information about FSMA and the FSSC FSMA Addendum below.
- What is FSMA?
The widely used acronym: “FSMA”, refers to the US FDA’s Food Safety Modernization Act, which was enacted in early 2011. Written in the wake of a number of major food safety incidents the law is aimed at reducing the number of preventable food safety incidents in the US that can significantly impact public health, and which “pose a threat to the economic well-being of the US food system” www.FDA.gov/food.
Comprised of 7 individual regulations FSMA takes a preventive approach to protecting the US food supply chain by placing the responsibility of controlling potential food safety hazards on to the producer, manufacturer, shipper or importer (See Table 1). As hazards can be introduced at any part of the supply chain, suppliers of ingredients or raw materials as well as finished products are a potential contamination risk to the US food supply.
Table 1: The 7 Rules of FSMA and the different sectors of the supply chain to which they apply.
| Regulation: | Also known as: | Applies to: |
| Preventive Controls Rule for Human Food (PCHF) | All non-exempt manufacturers of human marketing in the US | |
| Preventive Controls Rule for Animal Food (PCAF) | All non-exempt manufacturers of human marketing in the US | |
|
Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption |
The Produce Rule | All non-exempt growers of fresh produce being marketed in the US |
| Foreign Supplier Verification Programs | FSVP | All US importers of food stuffs, not already covered by the Preventive Controls Rule |
| Mitigation Strategies to Protect Food Against Intentional Adulteration
|
Food Defense or IA Rule | All manufactures/ producers of food marketed in the US |
| Sanitary Transportation of Human and Animal Food
|
Sanitary Transport Rule | All transporters (Road/ Rail) in the US |
| Accredited Third-Party Certification and Voluntary Qualified Importer Program |
TPP VQIP |
Any suppliers wishing to take advantage of a voluntary expedited entry into the US |
From <www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/food-safety-modernization-act-fsma>
- The international Impact of FSMA
While there are exemptions for some, and modified requirements for others, the FDA FSMA rules apply to all “facilities engaged in manufacturing, processing, packing, or holding food for consumption in the United States”. This means that any company producing or supplying foodstuffs for the US market is subject to the regulations, whether they are located within the US as a domestic manufacturer, an importer receiving goods from overseas or a foreign supplier providing ingredients or finished products for sale in the US. It is this aspect of the FSMA law that extends its reach beyond the borders of the USA and gives what was originally considered a US-centric rule, international impact.
All facilities that market foodstuffs in the US are required to register with the FDA and this applies to both foreign and domestic facilities. All registered facilities must renew their registration every other year and under the revised FSMA regulations agree to allow the FDA to inspect the facility at their request to ensure that the requirements of FSMA are being met. Thus, any facility supplying the US must be prepared for an obligatory FDA inspection and be able to demonstrate how it is meeting all the requirements.
Exemptions, and modified requirements do apply to facilities already subject to the existing legislation like Seafood HACCP, juice HACCP or LACF HACCP. Detailed FDA guidance on exemptions and how the rules apply to these already regulated producers is available on the FDA website ( www.FDA.gov/food).
- The Key Elements of the Preventive Controls Rules
The backbone of FSMA is the “Preventive Controls Rule”. It defines the basic requirements for manufacturers, processors, and their suppliers and requires that all facilities have a written food safety plan that includes a thorough hazard analysis for their process or function. All known or reasonably foreseeable biological, chemical, and physical hazards that might impact their product must be identified and prevented or minimized using specific Preventive controls.
While the requirement for food safety management through risk management and hazard control is not new to facilities familiar with FSSC 22000, FSMA introduces some new terminology such as the use of “preventive controls” which need to be identified, documented and used to control any hazards identified. These include Process Controls, Allergen, and Sanitation controls, as well as Supply-Chain Controls. Facilities must also have a recall plan, management oversight and competent qualified individuals that are responsible for the preparation and implementation of food safety. FSMA uses the term Preventive Control Qualified Individual (PCQI) to identify the responsible persons. Verification activities are also described in the regulation and required to ensure that the preventive controls are implemented and effective through monitoring and evaluation, and everything needs to be documented, along with any corrective actions and corrections and the documentation made available for inspection.
www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-preventive-controls-human-food
- Why the FSSC 22000 FSMA Addendum?
The need for hazard control and exemplary food safety management is a major component of the food safety management system of FSSC 22000 and one of the most frequently asked questions is always “If I am FSSC 22000 certified, do I meet the requirements of FSMA?” To help companies answer this question for their own situation, FSSC 22000 has published the updated alignment document and produced the FSSC 22000 FSMA Addendum.
There are many parallels between FSSC 22000 and the FSMA Preventive Controls Rules which help companies achieve compliance. Both require a preventive approach to Risk Based Hazard Control aligned with CODEX HACCP. Both have an international impact through a Food Safety Management approach which applies to the entire supply chain. Both require interactive communication with suppliers and customers, and both focus on continuous improvement. Like FSSC 22000, FSMA was developed to provide a robust program to manage food safety, but also to remain flexible enough to apply to many situations. Both FSSC 22000 and FSMA result in domestic and foreign parity with FSSC 22000 providing a recognized global standard that can be used by companies all over the world.
The 2020 alignment shows how similar the two approaches are and how the revision of ISO 22000:2018 has helped close many previously identified gaps. It explains how the prerequisite programs (PRP) and operational prerequisite program (OPRP) requirements of ISO 22000 translate to the preventive control requirements of FSMA (Table 2) and contribute to the food safety plan. Certified companies can also use the document to conduct their own gap analysis against the regulations to help themselves in achieving compliance.
Table 2 Comparison of the Terms used by FSMA, HACCP and FSSC 22000
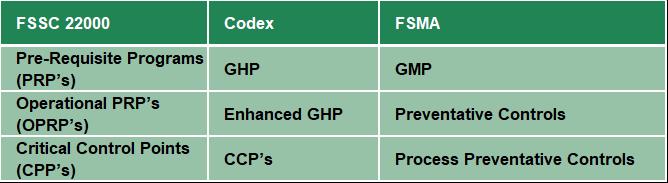
There are some nuances to the FSMA legislation that are highlighted, such as the use of terminology already described and the need to identify the preventive controls that are being applied. There is a call for greater detail in some sections of the regulation relating to the role of microbiological testing in environmental monitoring and a regulatory requirement for more transparency within the supply chain through the FSMA Supply chain control. These are included and summarized in the addendum.
- Supplier Verification
Transparency is a major part of the Supplier Verification requirement of the FSMA defined supply chain program. Verification activities described in the PCHF and FSVP rules require a manufacturer or importer to conduct a risk analysis and include the option of using an annual onsite third-party audit to verify adequate control of any identified potential hazards by the supplier.
In order to support the use of FSSC 22000 as an applicable audit, the FSSC 22000 FSMA Addendum includes an the FSSC 22000V5 FSMA PCHF Report Addendum which should be completed by a qualified auditor from an FSSC 22000 licensed Certification Body, at the time of the FSSC 22000 annual audit. It can be shared within supply chain, accompanying the audit report, and is a declaration of awareness of the PCHF requirements, confirmation of the hazards being controlled and details of the preventive controls being used. It is this information that is required by customer companies and US based importers to complete their own supplier verification activities.
- Who Recognizes the FSSC 22000 FSMA Addendum?
While the FSSC 22000 FSMA Addendum does not confer FSMA compliance, or substitute for an FDA inspection or audit, it could be used to support the role of an FSSC 22000 audit in contributing to supplier verification and be submitted for review by the customer, or importer, as part of their supply chain control verification requirement of foreign (and domestic) suppliers.
An onsite audit is listed as one of the verification activities that a U.S. Food and Beverage Importer may perform to verify that the food they import is safe and that it is produced in a manner that meets the statutory safety outcomes as outlined in FSMA. The FSSC 22000 Report Addendum shows how a company has integrated the requirements of the FSMA Preventive Controls Rule into their Food Safety Management System (FSMS). In doing so it can be used by the importer to fulfil their own obligations of the Preventive Controls Rule, or FSVP by contributing to the verification activities.
www.fda.gov/food/food-imports-exports/importing-food-products-united-states
Other Verification activities of foreign suppliers include:
- Monitoring shipment records that might reveal the food is safe,
- Conducting annual on-site inspections of the foreign supplier
- Obtaining a copy of and evaluating the Foreign Supplier’s HARPC Plan or records proving food complies with applicable Standards of Produce Safety
- Lot-by-lot food certification of compliance
- Periodic sampling and testing shipments challenging manufacturer Certificates of Analyses
FSVP Regulation Records Requirements List
- How many companies are potential clients for FSSC 22000 V5 FSMA PCHF Addendum?
Any company that produces for the US market and is certified by FSSC 22000 certified is a potential client for the FSSC 22000 V5 FSMA PCHF Addendum. An indication of the number of potential users can be gained from the latest figures of the FDA registered facilities. Under FSMA, food facilities are required to renew their FDA registrations between October 1 and December 31 of each even-numbered year. Recent data published by Registrar Corp and reported by Food Safety News reveals that as of Dec. 31, 2019, there were 221,843 FDA-registered food facilities, with 127,420 of them, or 57 percent, located outside of the United States.
Compared to similar reports Registrar Corp. has received in the previous three years, the data illustrates a gradual increase of 14,190 or 7 percent registered food facilities between December 2016 and December 2019 and an increase of 24% non US facilities.
The table below shows an up to date comparison of the number of domestic and foreign registered facilities.
| FDA Registered Food Facilities (January 2018 – December 2019) (Percentages represent changes from the previous period) |
||||
| Jan 2018 | Dec 2018 | Feb 2019 | Dec 2019 | |
| U.S. Registrations | 93,871 (+17.40%) | 102,207 (+8.88%) | 82,921 (-18.87%) | 94,423 (+13.87%) |
| Non-U.S. Registrations | 115,647 (+62.95%) | 131,444 (+13.66%) | 103,103 (-21.56%) | 127,420 (+23.59%) |
| Total Registrations | 209,518 (+39.74%) | 233,651 (+11.52%) | 186,024 (-20.38%) | 221,843 (+19.26%) |
As of December 2019, the five countries with the most FDA-registered food facilities are China, Japan, France, Italy, and Mexico. These countries are home to 25 percent of all FDA-registered food facilities and 44 percent of all non-U.S. registered facilities.
According to the Office of the United States Trade Representative, The United States has trade relations with more than 75 countries around the world. https://ustr.gov/countries-regions
The top five export markets for U.S. goods in 2017 were:
- Canada, $282 billion
- Mexico, $243 billion
- China, $130 billion
- Japan, $68 billion
- United Kingdom, $56 billion
Any company that manufactures or processes food for the US market is a potential client that can benefit from the FSSC 22000 FSMA addendum when accompanied by FSSC 22000 Certification. In addition, those seeking to be aware of the requirements of FSMA and to assure their customers within their supply chain of their capacity to control any applicable hazards through good food safety management would also benefit both from FSSC 22000 certification and the accompanying FSSC 22000 V5 FSMA Addendum.
References
www.fda.gov
Disclaimer
The information provided in this document is strictly for information only. It does not constitute legal or regulatory advice. FSSC 22000 makes no warranties as to the accuracy or completeness of the information.



